Our research focuses on numerical methods, computational models, and digital control solutions for neural prosthesis. Our mission is to develop and validate intelligent systems for adaptive neural stimulation, personalized treatments for neural disorders, and assistive tools for neurological impairments. Our research combines stochastic modeling, machine learning, dynamical systems, control theory, and neural data (e.g., EEG, spike trains, calcium images, etc.), and it spans several applications, including Parkinson’s disease, dystonia, epilepsy, brain-computer interface, and perception. A partial list of research areas and projects we are currently working on is reported below:
Deep Brain Stimulation and Parkinson’s Disease![By Shamir R, Noecker A and McIntyre C [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons](https://nsec-lab.media.uconn.edu/wp-content/uploads/sites/1339/2016/08/PictureDBS-276x400.png)
Deep Brain Stimulation (DBS) of the basal ganglia and thalamus is clinically recognized to treat movement disorders in Parkinson’s disease (PD) but the mechanisms remain elusive. Hypotheses developed thus far rely on the assumption that the neural loop targeted by DBS is an hierarchical system of individual structures that process motor-related information in a strictly sequential, feed-forward way. It is also assumed that DBS works locally in the region where the stimulus is injected and its effects eventually drive the neural activity in the structures downstream of this region, thus masking the ongoing pathophysiologic activity. None of these hypotheses, however, seems satisfactory. Our work provide experimental evidence that challenges these premises, and document the development of a computational framework to analyze the entire neural loop under DBS. Through our work, a novel hypothesis is proposed, which explains a wider array of (apparently contradictory) experimental evidences from PD patients and animal models of PD under DBS.
Image by Shamir R, Noecker A and McIntyre C [CC BY 3.0 (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons
Seizure Onset Detection in Epilepsy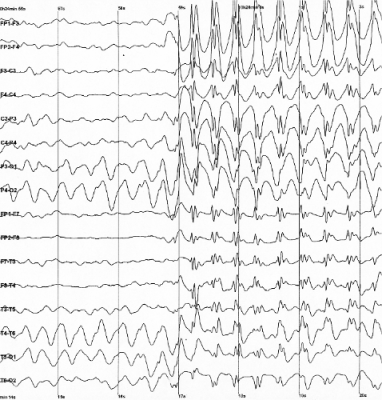
We are currently focused on developing a computational framework for online hidden state transition detection from multi-channel physiological time series, with application to the unsupervised detection of seizures in patients with drug-resistant epilepsy (DRE). The framework combines stochastic chain modeling, Bayesian estimation, and optimal control, and it is applied to multi-channel, multi-day intracranial recordings from DRE patients. Differently from existing seizure detection paradigms, the proposed framework introduces a cost function to account for the desired performance goals, and derives the detection policy such that the cost is minimized. In this way, the expected performance is stated upfront and the detection policy is derived accordingly. Our approach aims to tackle the lack of robustness and optimality that often have been found in current paradigms for seizure detection community.
Image by Der Lange [CC BY 2.0 (http://creativecommons.org/licenses/by-sa/2.0/deed.en)], via Wikimedia Commons
Seizure Onset Zone Localization in Epilepsy
Patients with partial epileptic seizures have focal regions that periodically diverge from normal brain network dynamics during seizures. We are interested in characterizing the evolution of the brain connectivity before, during, and after seizures, and to investigate graph-theoretic features of the intracranial EEG signals that may uniquely identify the epileptogenic onset zone. Our work has showed that the brain network connectivity defines a finite set of brain states and that seizures are characterized by a consistent state progression. The sequence of states visited during the progression is patient-specific and consistent across seizures. We are now investigating whether EEG-based network metrics can be used to localize the seizure onset zone and to study the dynamics of such zone as it diverges from normal overall network structure.
Image available under CC0