Deep Brain Stimulation and Parkinson’s Disease
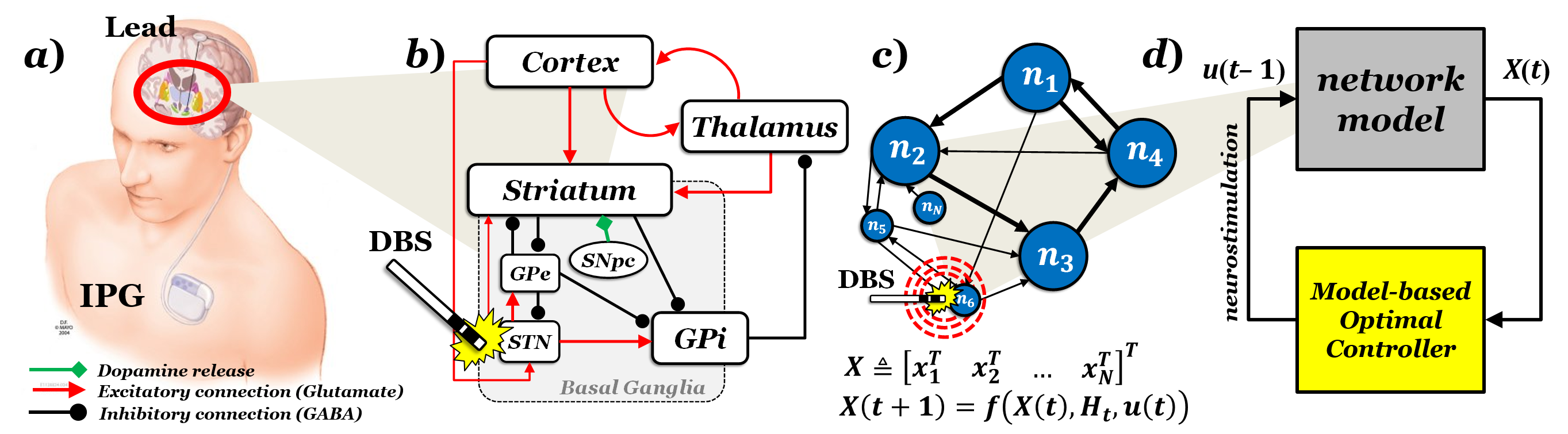
Parkinson’s disease (PD) is the second most common age-related neurological disease, with 10 million patients world-wide and 2 million in the US. The risk of developing PD dramatically increases with age. Approximately 95% of the PD patients are over the age of 60 and PD patients account for 1% of the population worldwide over the age of 60 and 5% of the population over the age of 85. Deep brain stimulation (DBS) is a clinically recognized treatment for drug-resistant PD patients but its cellular and network mechanisms remain elusive, and this has limited the overall development and penetration of the treatment. Performance would highly increase and setup procedures would be faster if a clear rationale for the expected effects of DBS would be available. Current approaches look at the effects that DBS locally elicits in the target site and assume that such effects may predict the therapeutic output. However, growing evidence in humans and animal models of PD suggests that DBS likely affects the dynamics of a spatially distributed neural network (i.e., the motor loop) and can hardly be explained by looking at local effects.
This program focuses on developing a novel computational framework to study the mechanisms of DBS and to design optimal DBS therapies. The framework includes modeling the network dynamics of the structures targeted by DBS, i.e., representing the motor loop as a network of interconnected, re-entrant, and overlapping neuronal circuits; and characterizing the global effects of DBS on the motor loop, i.e., representing the perturbations evoked by DBS both orthodromically and antidromically as a coordinated ensemble of time-sensitive, spatially-distributed perturbations that enter multiple nodes in the network simultaneously. Two hypotheses are investigated: (1) the therapeutic effects of DBS stem from an overall restoration of the network function rather than from local changes in the neural activity in individual structures (e.g., increased regularity of the discharge patterns or suppression of beta band oscillations), and (2) changes in the neural dynamics of the motor striatum may be pivotal to the network-wide effects of therapeutic DBS and can be used as a proxy of the overall restoration of the loop function. To this purpose, a computational test-bed is constructed to explicitly simulate the neural activity of every node in the motor loop and to closely reproduce discharge patterns and population-wide dynamics reported for each and every node in healthy and PD conditions, with and without DBS. Furthermore, the relationship between DBS settings, target site, and striatal activity are modeled and used in combination with optimization tools to design innovative DBS therapies (i.e., stimulus profile, frequency, and target). These therapies aim to minimize a penalty function that accounts for the level of network restoration, the amount of power used, and safety constraints.
Publications produced as a result of this research
- Santaniello S, McCarthy MM, Montgomery EB Jr., Gale JT, Kopell N, Sarma SV (2015) “Therapeutic Mechanisms of High Frequency Stimulation in Parkinson’s Disease and Neural Restoration via Loop-based Reinforcement,” Proc. Nat. Acad. Sci. USA, vol. 112(6):E586-95. DOI: 10.1073/pnas.1406549111
- Santaniello S, Montgomery EB Jr., Gale JT, Sarma SV (2012) “Non-stationary Discharge Patterns in Motor Cortex under Subthalamic Nucleus Deep Brain Stimulation,” Front. Integr. Neurosci., vol. 6:35. DOI: 10.3389/fnint.2012.00035
- Santaniello S, Fiengo G, Glielmo L, Grill WM (2011) “Closed-Loop Control of Deep Brain Stimulation: A Simulation Study,” IEEE Trans. Neural Syst. Rehabil. Eng., vol. 19(1):15-24. DOI: 10.1109/TNSRE.2010.2081377
- Santaniello S, Fiengo G, Glielmo L, Catapano G (2008) “A Biophysically Inspired Microelectrode Recording-based Model for the Subthalamic Nucleus Activity in Parkinson’s Disease,” Biomed. Signal Process. & Control, vol. 3(3):203-11. DOI: 10.1016/j.bspc.2008.03.001
- Agarwal R, Santaniello S, Sarma SV (2014) “Generalizing Performance Limitations of Relay Neurons: Application to Parkinson’s Disease,” 36th IEEE Annual Conference of the EMBS (EMBC). Chicago, IL, pp. 6573-6. DOI: 10.1109/EMBC.2014.6945134
- Santaniello S, Gale JT, Montgomery EB Jr., Sarma SV (2012) “A Point Process Model-Based Framework Reveals Reinforcement Mechanisms in Striatum During High Frequency STN DBS,” 51st IEEE Conference on Decision and Control (CDC). Maui, HI, pp. 1645-50. DOI: 10.1109/CDC.2012.6426098
- Santaniello S, Gale JT, Montgomery EB Jr., Sarma SV (2012) “Reinforcement Mechanisms in Putamen during High Frequency STN DBS: A Point Process Study,” 34th IEEE Annual Conference of the EMBS (EMBC). San Diego, CA, pp. 1214-7. DOI: 10.1109/EMBC.2012.6346155
- Pedoto G, Santaniello S, Fiengo G, Glielmo L, Hallett M, Zhuang P, Sarma SV (2012) “Point Process Modeling Reveals Anatomical Non-Uniform Distribution across the Subthalamic Nucleus in Parkinson’s Disease,” 34th IEEE Annual Conference of the EMBS (EMBC). San Diego, CA, pp. 2539-42. DOI: 10.1109/EMBC.2012.6346481
- Pedoto G, Santaniello S, Montgomery EB Jr., Gale JT, Fiengo G, Glielmo L, Sarma SV (2010) “Analyzing Local Field Potentials in the Healthy Basal Ganglia under Deep Brain Stimulation,” 49th IEEE Conference on Decision and Control (CDC). Atlanta, GA, pp. 6064-8. DOI: 10.1109/CDC.2010.5717450
- Santaniello S, Gale JT, Montgomery EB Jr., Sarma SV (2010) “Modeling the Effects of Deep Brain Stimulation on Sensorimotor Cortex in Normal and MPTP Conditions,” 32nd IEEE Annual Conference of the EMBS (EMBC). Buenos Aires, Argentina, pp. 2081-4. DOI: 10.1109/IEMBS.2010.5626285
- Santaniello S, Gale JT, Montgomery EB Jr., Sarma SV (2010) “Modeling the Motor Striatum under Deep Brain Stimulation in Normal and MPTP Conditions,” 32nd IEEE Annual Conference of the EMBS (EMBC). Buenos Aires, Argentina, pp. 2065-8. DOI: 10.1109/IEMBS.2010.5626354
- Saxena S, Santaniello S, Montgomery EB Jr., Gale JT, Sarma SV (2010) “Point Process Models Show Temporal Dependencies of Basal Ganglia Nuclei under Deep Brain Stimulation,” 32nd IEEE Annual Conference of the EMBS (EMBC). Buenos Aires, Argentina, pp. 4152-5. DOI: 10.1109/IEMBS.2010.5627350